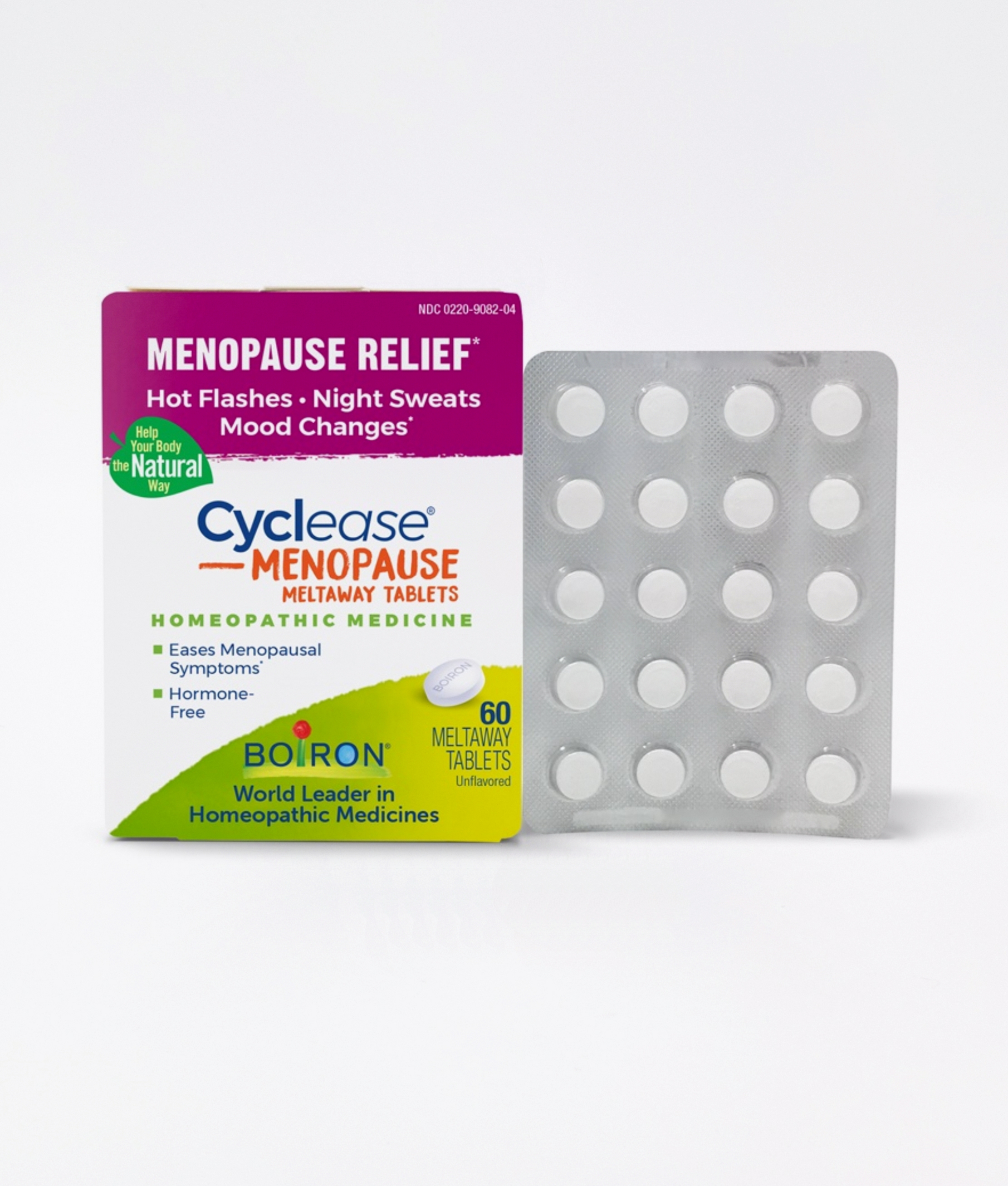
Boiron Cyclease® Menopause Tablets
$12.49
Relieves hot flashes, night sweats and irritability associated with menopause and pre-menopause. Ideal for women looking for an alternative to HRT (Hormone Replacement Treatment).
Available in a box of 60 slow-dissolving tablets.
Always secure shopping.

MULTI-SYMPTOM FORMULA: Cyclease Menopause Tablets is designed to address several symptoms of menopause and pre-menopause including night sweats, mood changes, irritability, occasional sleeplessness, and a red, blotchy, or flushed face associated with hot flashes. Clinically supported to reduce hot flashes and successfully used in Europe for nearly a decade.
WOMEN FRIENDLY: Menopausal and premenopausal women, who do not qualify for hormonal therapy or cannot take phytoestrogens, can now find hot flash relief with Cyclease Menopause Tablets. It does not cause drowsiness or interact with other medications, and it is not contraindicated with pre-existing conditions. It is hormone- and soy-free.
SHORTEN YOUR SYMPTOMS: Cyclease Menopause Tablets work best when taken daily for three months.
HOMEOPATHIC MEDICINE: Cyclease Menopause Tablets use highly diluted biological, botanical, or mineral substances to relieve symptoms. It works with your body without the risk of contraindications, or known drug interactions.
THE BOIRON PROMISE: We believe there’s more than one way to feel better. Since 1932, we have been committed to providing quality medicines. As a world leader in homeopathy, our passion is your health. Our promise is your satisfaction.
Temporarily relieves hot flashes, night sweats and irritability associated with menopause and pre-menopause.
- A homeopathic medicine that works naturally with your body
- Non-drowsy; No side effects; No drug interactions; No contraindications
- Hormone-free; Soy-free; Alternative to HRT (Hormone Replacement Treatment)
- For best results, use daily for three months
- Regulated as a OTC (Over the Counter) drug by the FDA
- Used in more than 50 countries, for more than 50 years
- Available in a box of 60 slowly-dissolving tablets
- Adults and children 12 years of age and older: At the onset of symptoms, day or night, allow 1 tablet to dissolve slowly in the mouth up to 4 times in 24 hours. For best results, daily use for three months is recommended.
- Children under 12 years of age: Not recommended.
- Stop use and ask a health professional if symptoms persist for more than 7 days or worsen.
- Keep out of reach of children.
- If pregnant or breast-feeding, ask a health professional before use.
STORAGE & SAFETY:
- Do not use if glued carton end flaps are open or if the tray seal is broken.
- Store at 68-77°F (20-25°C).
Active Ingredient:**
- Arnica montana 4C HPUS — relieves blotchy face associated with hot flashes
- Cimicifuga racemosa 4C HPUS — relieves irritability and occasional sleeplessness associated with menopause
- Glonoinum 4C HPUS — relieves sudden hot flashes with profuse sweating and throbbing headache associated with menopause
- Lachesis mutus 5C HPUS — relieves irritability, hot flashes and night sweats associated with menopause
- Sanguinaria canadensis 4C HPUS — relieves flushing of the face associated with menopause
The letters HPUS indicate that this ingredient is officially included in the Homeopathic Pharmacopœia of the United States.
Inactive Ingredients:
- Sucrose
- Lactose
- Magnesium stearate
**C, K, CK, and X are homeopathic dilutions. Learn More.
Boiron was founded in 1932 in Lyon, France, by twin brothers and pharmacists Jean and Henri Boiron. In 1983, Boiron established a U.S. presence by acquiring John A. Borneman & Sons, Inc. in suburban Philadelphia. Today, Boiron USA has evolved into a leading U.S. homeopathic medicine supplier. The company partners with brokers, distributors and retail chains to make homeopathic medicines available to a wide variety of consumers and physicians. The Boiron Group has a strong sense of obligation to respect the environment. The preparation of their medicines has a limited impact on the environment. The main effects derive from extracting raw materials (continuity of species gathered), water pollution (mainly organic and biodegradable) and waste. As a counterbalance, Boiron promotes re-supplying wild or organically-farmed plants when possible. This sequentially improves the quality of raw materials used.
| Weight | 0.02 lbs |
|---|---|
| Dimensions | 1.2 x 3 x 4 in |
Only logged in clients who have purchased this product may leave a review.
Refer-A-Friend Program (see here for more details)
Get started now, share your referral link with your friends and family!
Share via
Related products
Homeopathic Meds
All-Natural Supplements
Homeopathic Meds
Homeopathic Meds
All-Natural Supplements
Homeopathic Meds
Homeopathic Meds
Homeopathic Meds
































Reviews
There are no reviews yet.